“Fertility is not just a female issue. It’s a man’s too. I ordered for my husband.”
Davrex Study
Evaluating the Effectiveness of Davrex Testicular Thermoregulation Device in Maintaining Optimal Testicular Temperature During Prolonged Sitting: An Internal Pilot Study
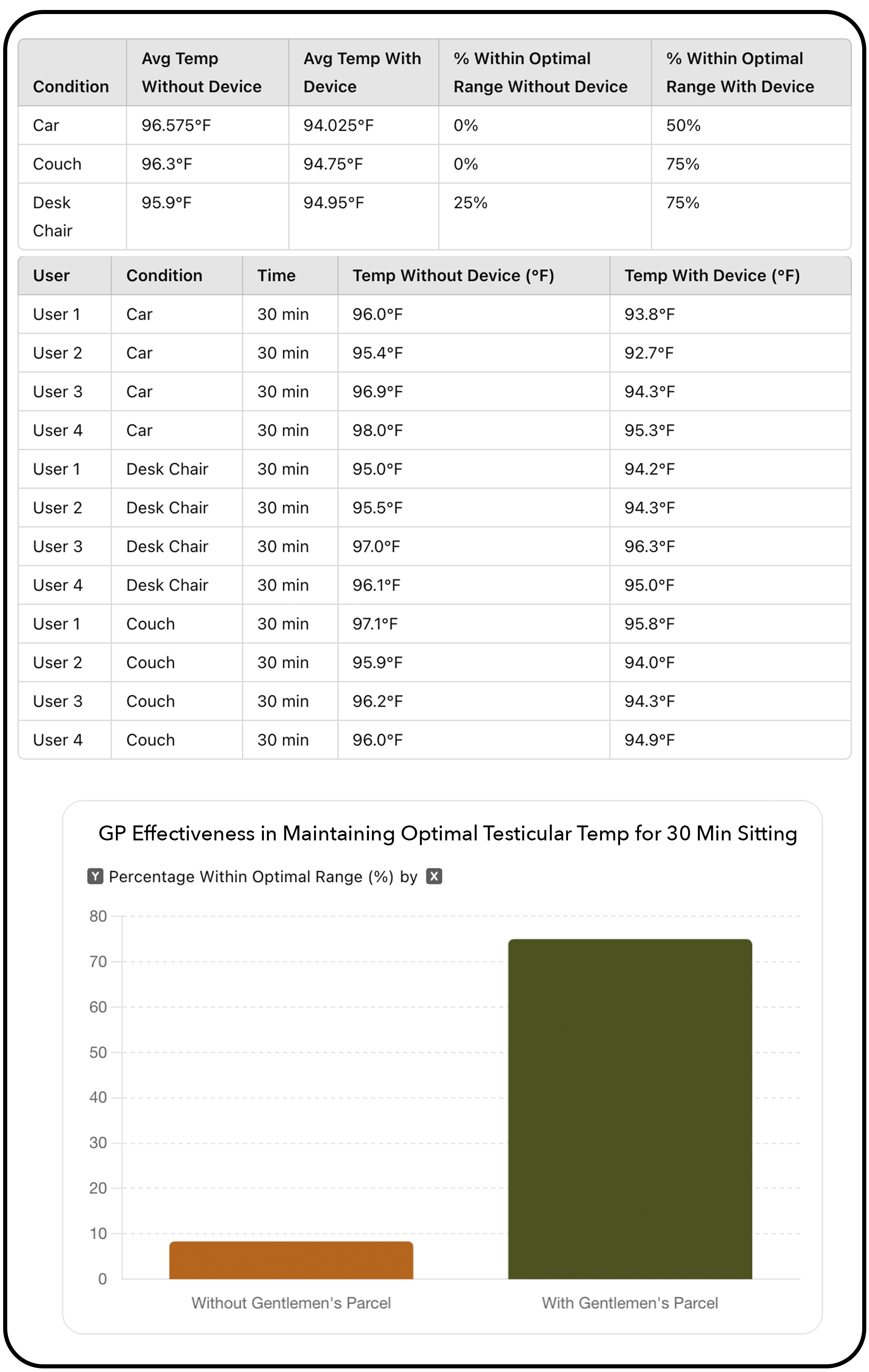
Abstract: This study evaluates the effectiveness of the Davrex testicular thermoregulation device in maintaining optimal testicular temperature during prolonged sitting. The device successfully brought test-users' scrotums back into the optimal temperature range 75% of the time, compared to being out of range over 90% of the time without it. The findings suggest significant benefits for male fertility and comfort, highlighting the device's potential in reproductive health management. The average cooling effect was 1.68°F. Normal body temperature is 98.6°F, while functioning testicular temperature should be 93°-95°F.
Data With and Without Device:
After 30 minutes of sitting in a car, on a desk chair, and on a couch (not including cooling pod therapy), test-users 1 and 2 wore boxer briefs, while test-users 3 and 4 wore boxer shorts. Test-users 1 and 3 wore pants, and test-users 2 and 4 wore shorts. Testing environments were maintained as close as possible to 72°F. All participants, aged in their 30’s and 40’s, were of healthy weight and without any health conditions. An ear thermometer was used for temperature measurements. Participants engaged in instructional tutorials on how to use the device and how and when to take measurements accurately. All measurements were conducted by the participants themselves.
Conclusion:
This internal pilot study demonstrates the Gentlemen’s Parcel device's significant potential in regulating scrotal temperature, bringing it back to the optimal range in 75% of cases. While these initial findings are promising, further testing with larger participant groups and comprehensive pre- and post-sperm analysis is needed to fully understand the device's impact on fertility and overall testicular health.
The Science…
Men’s testicles evolved to hang away from the body to stay cool—because without proper cooling, they fail to perform.
For optimal function, testicular temperature must remain 3° to 4°F cooler than the rest of the body to support sperm production, testosterone synthesis, and hormonal balance. The scrotum is uniquely designed for this regulation, with a higher concentration of sweat glands to help manage temperature.
You Are Not Alone: The Growing Male Fertility Crisis
Male infertility has surged by 77% in recent decades. Men today have half the sperm count their grandfathers did—yet male reproductive health remains largely ignored. Infertility affects 10% of men globally, yet only 25% of men are tested or treated during fertility evaluations (Kumar & Singh, 2015).
Prolonged Sitting Elevates Groin Temperatures:
With sedentary jobs increasing by 83% since 1950, many men now spend over 70% of their waking hours sitting (Owen et al., 2010). This prolonged sitting raises scrotal temperatures beyond optimal levels, impairing sperm production and testosterone (Figà-Talamanca et al., 1996). As a result, sperm counts have dropped by 50% in the last 40 years (Aitken & Krausz, 2001), and male infertility rates have surged by 77% (Ji et al., 1997). The testicles, designed to hang outside the body to stay cool, are increasingly exposed to overheating—contributing to the modern fertility crisis.
Healthy sperm leads to healthy pregnancies and children:
Men with poor-quality sperm and high levels of sperm DNA fragmentation double the likelihood of a miscarriage. Sperm DNA damage has been linked to numerous diseases and disorders in offspring, including autism, epilepsy, and childhood cancer, as shown in studies by Ji, Aitken, Krausz, Sipos, Vestergaard, and Reichenberg.
Male fertility is a women’s health issue:
Despite men being responsible for half of all infertility cases, 25% of men are not tested or treated during infertility exams (Kumar & Singh, 2015). Women carry most of the treatment burden even when male issues like low sperm count, reduced motility, or abnormal morphology are identified (Agarwal et al., 2015). This must change.
Poor sperm quality has been linked to decreased maternal health:
It contributes to an increased risk of preeclampsia—a severe complication causing high blood pressure and organ damage in mothers. Abnormal sperm DNA can impair placental development, leading to complications like restricted fetal growth or gestational diabetes. (Mansour & Elfayomy, 2020; Aitken et al., 2014; Kumar et al., 2020)
Testicular health is directly linked to overall male health.
Low sperm quality has been identified as a biomarker for increased risks of cardiovascular disease, cancer, diabetes, and reduced life expectancy (Eisenberg et al., 2014; Ferlin et al., 2021). Research shows that addressing testicular health can have broader implications for long-term male health and longevity.
“We wanted to take every precaution for a healthy pregnancy.”
Testicular cooling studies prove cooling therapy works:
Among studies published in the Journal of Urology, Dr. Mulcahy showed that sperm density and motility doubled in 65% of his patients after applying ice packs to the scrotal area at night (Mulcahy, 1984). Dr. Jung discovered similar significant increases in sperm count and concentration from nocturnal scrotal cooling (Jung et al., 2008). Dr. Lavern demonstrated that men who slept and worked in cooler scrotal conditions had significantly higher levels of motility compared to those in warmer environments (Lavern et al., 2011)
Testicular Overheating in Male Athletes: Hidden Risks to Fertility & Health
Intense physical activity and overheating significantly impact male fertility. A Human Reproduction study found professional soccer players experienced longer conception times and a higher likelihood of conceiving female offspring due to heat-sensitive male sperm (Oxford Academic). Cyclists riding over five hours a week had a 40% reduction in sperm count and motility from scrotal pressure and heat (Wise, L.A., et al., 2010). Other sports like triathlons and rowing also raise scrotal temperatures, causing DNA damage and impaired sperm quality (Setchell, B.P., et al., 2002; Mieusset, R., et al., 1987).
Commercial drivers have abnormally low sperm counts, motility, and quality:
One study showed taxi drivers have almost 20% fewer normal sperm forms and longer times to conceive (Figà-Talamanca et al., 1996). Another study revealed rapid scrotal temperature increases while driving: from 34.2°C to 35.5°C in the first 20 minutes, and to 36.2°C over two hours (Bujan et al., 2000). Functional scrotal temperature is between 32-35°C; anything higher harms sperm, alters DNA, and affects testosterone and overall health (Mulcahy, 1984). This has significant implications for all men who are seated and overheated during the workday. In the sitting position, testicles are trapped and unable to extend away from the body to cool properly.
Testosterone: The Key to Male Vitality
Davrex supports natural testosterone production through cooling therapy, maintaining the optimal testicular temperature range (31–36°C) shown to boost testosterone levels (Nakamura et al.). Overheated testicles significantly reduce testosterone synthesis, even in short periods.
Testosterone is vital for male health, regulating libido, muscle mass, bone density, fat distribution, red blood cell production, and sperm. With 95% of testosterone produced in the testicles, optimal testicular health is essential for vitality.
Testosterone and sperm production are interdependent, both stimulated by the same precursor hormones—Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH). Impaired testicular function disrupts both processes, highlighting the importance of maintaining testicular health (Saez, J.M., 1994; Andrology Journal).
While testosterone supplements are popular, side effects include infertility, testicular atrophy, prostate enlargement, and cardiovascular risks (Brady et al., 2006; Mayo Clinic, 2024). Studies show 65% of men taking supplements experience azoospermia within four months, increasing to 95% in six months.
Davrex provides a safe, natural alternative to optimize testosterone and sperm production, aligning both for healthier, more virile men.
“Even though we can’t conceive naturally, I still want the best specimen for IVF.”
Conceive Naturally or Through IVF
Many believe men don’t have a biological clock, but sperm count and quality decline after age 35. In recent decades, we’ve seen a rise in low testosterone, poor sperm quality, and infertility across all ages.
While many couples hope to conceive naturally, IVF can be a life-changing option—but sperm health remains a critical factor for success. Even with advanced technology, IVF requires healthy, motile, and well-formed sperm for fertilization. Poor sperm quality reduces IVF success rates, making testicular health essential regardless of the path to conception.
Prioritizing optimal sperm health today increases the chances of natural conception and successful fertility treatments. (Zhao et al., 2023)
Consult Your Doctor
Infertility is complex and varies from person to person. We recommend consulting your doctor, urologist, or fertility specialist to determine the best approach for you.
At the very least, Davrex can complement your fertility journey—but for many, cooling therapy was the breakthrough that made the difference. After exhausting other options, testicular cooling was what ultimately reversed their infertility.
Why not start with space, airflow, and cooling therapy—proactively optimizing your chances before facing a difficult, emotional path? Davrex, combined with targeted supplements, a science-backed lifestyle guide, a healthy diet, and exercise, should be the first step when planning for a family.
Medical & Scientific Studies
Supporting Cooling Therapy
and a Healthy Testicular Environment
to Improve Fertility, and the Health of the Child, Father, and Mother.
Further, your research with our list of studies from the medical and scientific community from over the past 50 years (in chronological order) supporting the effectiveness of testicular cooling and healthy testicular environment on sperm count, sperm quality, and testosterone.
Waites, G.M.H. and Voglmayr, J.K. (1963) The functional activity and control of the apocrine sweat glands of the scrotum of the ram. Aust. J. Agric. Res., 14, 839–851.
Rock, J. and Robinson, D. (1965) Effect of induced intrascrotal hyperthermia on testicular function in man. Am. J. Obstet. Gynecol., 93, 793–801.
National Center for Health Statistics, Rosenberg HM. Seasonal variation of births: United States, 1933–63. Vital and health statistics. Series 21. No. 9. Washington, D.C.: Government Printing Office, 1966. (PHS publication no. 1000.)
Robinson & Rock, 1967. Intrascrotal hyperthermia induced by scrotal insulation: effect on spermatogenesis. Journal of Obstetrics and Gynecology, 1967 Feb;29(2):217-23.
Robinson D, Rock J, Menkin MF. “Control of Human Spermatogenesis Intrascrotal Temperature.” JAMA. (1968); 204(4):290-297. doi:10.1001/jama.1968.03140170006002.
Fowler, D.G. (1969) The relationship between air temperature, scrotal surface area and testis temperature in rams. Aust. J. Exp. Agric. Anim. Husb., 9, 258–261.
Chowdhury, A. K., and E. Steinberger. "Early changes in the germinal epithelium of rat testes following exposure to heat." Journal of Reproduction and Fertility 22.2 (1970): 205-212
Zorgniotti, A.W. and MacLeod, J. (1973) Studies in temperature, human semen quality and varicocele. Fertil. Steril., 24, 854–863.
Zorgniotti AW, Seaflon A, Toth A: “Chronic scrotal hypothermia as a treatment for poor semen quality.” Lancet (1980), 315(8174):904-906.
Zorgniotti AW, Seaflon AI, Toth A: “Further clinical experience with testis hypothermia for infertility due to poor semen.” Urology (1982), 19(6):636-640.
Zorgniotti, A.W. (1982) Elevated scrotal temperature. II. Indirect testis and intrascrotal measurement for clinical and research use. Bull. N. Y. Acad. Med., 58, 541–544.
Brindley, G.S. (1982) Deep scrotal temperature and the effect on it of clothing, air temperature, activity, posture and paraplegia. Br. J. Urol. , 54, 49–55.
Mulcahy JJ: “Scrotal hypothermia and the infertile man.” The Journal of Urology (1984), 132:469-470.
Zorgniotti AW, Seaflon AI: “Scrotal hypothermia: new therapy for poor semen.” Urology (1984), 23(5):439-441.
Mieusset, R., Grandjean, H., Mansat, A. and Pontonnier, F. (1985) Inhibiting effect of artificial cryptorchidism on spermatogenesis. Fertil. Steril., 43, 589–574.
Mieusset et al, 1985. Inhibiting effect of artificial cryptorchidism on spermatogenesis. Journal of Fertility and Sterility, 1985 Apr;43(4):589-94.
Kurz, K.R. and Goldstein, M. (1986) Scrotal temperature reflects intratesticular temperature and is lowered by shaving. J. Urol., 135, 290–294.
Zorgniotti AW, Cohen MS, Seaflon I: “Chronic scrotal hypothermia: results in 90 infertile couples.” The Journal of Urology (1986), 135:944-947.
Lynch, R., et al. "Improved seminal characteristics in infertile men after a conservative treatment regimen based on the avoidance of testicular hyperthermia." Fertility and Sterility 46.3 (1986): 476-479.
Mieusset R, Bujan L, Mondinat C, et al. "Association of scrotal hyperthermia with impaired spermatogenesis in infertile men." Fertility and Sterility (1987); 48:1006–1011
Mieusset, R., Bujan, L., Mansat, A. et al. (1987) Effect of artificial cryptorchidism on sperm morphology. Fertil. Steril., 47, 150–155.
Mieusset, Roger, et al. "Hyperthermia and human spermatogenesis: enhancement of the inhibitory effect obtained by ‘artificial cryptorchidism’." International Journal of Andrology 10.4 (1987): 571-580.
Kaufman, D. G., and H. M. Nagler. "Specific nonsurgical therapy in male infertility." The Urologic Clinics of North America 14.3 (1987): 489-498.
Levine RJ, Bordson BL, Mathew RM, Brown MH, Stanley JM, Starr TB. Deterioration of semen quality during summer in New Orleans. Fertil Steril 1988; 49:900–7.
Laven, Joop SE, Michiel J. Haverkorn, and Rob SGM Bots. "Influence of occupation and living habits on semen quality in men (scrotal insulation and semen quality)." European Journal of Obstetrics & Gynecology and Reproductive Biology 29.2 (1988): 137-141.
Colter, G.H., Senger, P.L. and Bailey, D.R.C. (1988) Relationship of scrotal surface temperature measured by infrared thermography to subcutaneous and deep testicular temperature in the ram. J. Reprod. Fert., 84, 417–421.
Galil, K. A. A., and B. P. Setchell. "Effects of local heating of the testis on testicular blood flow and testosterone secretion in the rat." International Journal of Andrology 11.1 (1988): 73-85.
Chan, S. L. "Male Infertility: Diagnosis and Treatment." Canadian Family Physician 34 (1988): 1735.
Richard J. Levine, M.D., Ravi M. Mathew, M.S., C. Brandon Chenault, M.D., Michelle H. Brown, B.S.P.H., Mark E. Hurtt, Ph.D., Karin S. Bentley, Ph.D., Kathleen L. Mohr, B.S., and Peter K. Working, Ph.D. (July 5, 1990) Differences in the Quality of Semen in Outdoor Workers during Summer and Winter, N Engl J Med 1990;323:12-16 DOI: 10.1056/NEJM199007053230103 VOL. 323 NO. 1
Bedford, J. Michael. "Effects of elevated temperature on the epididymis and testis: experimental studies." “Temperature and environmental effects on the testis.” Springer US (1991). 19-32.
Waites, G.M.H. (1991) Thermoregulation of the scrotum and testis: studies in animals and significance for man. In Zorgniotti, A.W. (ed.), Temperature and Environmental Effects on the Testis. Plenum Press, New York, pp. 1–8.
Bonde, 1992. Semen quality in welders exposed to radiant heat. British Journal Independent Medicine, 1992, Jan., vol 49, pp 5-10.
Mieusset, R., Quintana-Casares, P., Sanchez-Partida, L.G. et al. (1992) Effects of heating the testes and epididymides of rams by scrotal insulation on fertility and embryonic mortality in ewes inseminated with frozen semen. J. Reprod. Fert. , 94, 337–343
Shafik, A. (1992) Testicular suspension as a method of male contraception: technique and results. Adv. Contrac. Deliv. Syst ., VII, 269–279.
Candas, V., Becmeur, F., Bothorel, B. and Hoft, A. (1993) Qualitative assessment of thermal and evaporative adjustments of human scrotal skin to heat stress. Int. J. Androl., 16, 137–142.
Lerchl A, Keck C, Spiteri-Grech J and Nieschlag E (1993) “Diurnal variations in scrotal temperature of normal men and patients with varicocele before and after treatment.” International Journal of Andrology 16, 195-200.
Mieusset, R. and Bujan, L. (1994). The potential of mild testicular heating as a safe, effective and reversible contraceptive method for men. Int. J. Androl. , 17, 186–191.
Mieusset R, Bujan L. Testicular heating and its possible contributions to male infertility: a review. International Journal of Andrology (1995); 18:169-184.
Figà-Talamanca I, Cini C, Varricchio GC, Dondero F, Gandini L, Lenzi A, Lombardo F, Angelucci L, Di Grezia R, Patacchioli FR. Effects of prolonged autovehicle driving on male reproduction function: a study among taxi drivers. Am J Ind Med. 1996 Dec;30(6):750-8. doi: 10.1002/(SICI)1097-0274(199612)30:6<750::AID-AJIM12>3.0.CO;2-1. PMID: 8914722.
Setchell, B.P. and Mieusset, R. (1996) Testis thermoregulation. In Hamamah, S. and Mieusset, R. (eds), Male Gametes Production and Quality. INSERM, Paris, pp. 65–88.
Thonneau, P., Ducot, B., Bujan, L. et al. (1996) Heat exposure as a hazard to male fertility. Lancet , 347, 204–205.
Bonde et al, 1998. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. The Lancet, 1998 Oct 10;352(9135):1172-7.
Thonneau et al, 1998. Occupational heat exposure and male fertility: a review. Journal of Human Reproduction, Volume 13, Issue 8, 1 August 1998, Pages 2122–2125.
Hjollund, Niels Henrik I., et al. "Diurnal scrotal skin temperature and semen quality." International Journal of Andrology 23.5 (2000): 309-318.
Louis Bujan, Myriam Daudin, Jean-Paul Charlet, Patrick Thonneau, Roger Mieusset, Increase in scrotal temperature in car drivers, Human Reproduction, Volume 15, Issue 6, 1 June 2000, Pages 1355–1357, https://doi.org/10.1093/humrep/15.6.1355
Jung A, Eberl M, Schill WB: “Improvement of semen quality by nocturnal scrotal cooling and moderate behavioral change to reduce genital heat stress in men with oligoasthenoteratozoospermia.” Reproduction (2001), 121:595-603.
Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001 Oct;122(4):497-506. doi: 10.1530/rep.0.1220497. PMID: 11570956.
Jung et al, 2001. Genital heat stress as a threat to male fertility. Andrologia, 33, 316-317.
Hjollund NHI, Storgaard L, Ernst E, Bonde JP and Olsen J (2002b) “Impact of diurnal scrotal temperature and semen quality.” Reprod Toxicol 16, 215-221.
Hjollund et al, 2002. The relation between daily activities and scrotal temperature. Reproductive Toxicology, May 2002, 16(3):209-214.
Power RE, Scanlon R, Kay EW, Creagh TA, Bouchier-Hayes DJ. “Long-term protective effects of hypothermia on reperfusion injury post-testicular torsion.” Scand J Urol Nephrol (2003); 37:456–460.
Sipos A, Rasmussen F, Harrison G, Tynelius P, Lewis G, Leon DA & Gunnell D. (2004) Paternal age and schizophrenia: a population based cohort study. BMJ 329, 1070.
Jung, A., et al. "Influence of the type of undertrousers and physical activity on scrotal temperature." Human Reproduction 20.4 (2005): 1022-1027.
Jung, A., Schill, W. -B., Schuppe, H. -C., ‘Improvement of semen quality by nocturnal scrotal cooling in oligozoospermic men with a history of testicular maldescent.’ International Journal of Andrology, vol. 28/2 (2005), pp. 93-98
Lewis SE, Aitken RJ. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005 Oct;322(1):33-41. doi: 10.1007/s00441-005-1097-5. Epub 2005 Nov 3. PMID: 15912407.
Jung A, Schill WB, Schuppe HC: “Improvement of semen quality by nocturnal scrotal cooling in oligozoospermic men with a history of testicular maldescent.” International Journal of Andrology (2005), 28:92-98.
Borini, N. Tarozzi, D. Bizzaro, M.A. Bonu, L. Fava, C. Flamigni, G. Coticchio, Sperm DNA fragmentation: paternal effect on early post-implantation embryo development in ART, Human Reproduction, Volume 21, Issue 11, 1 November 2006, Pages 2876–2881
Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, Rabinowitz J, Shulman C, Malaspina D, Lubin G, Knobler HY, Davidson M & Susser E. (2006) Advancing paternal age and autism. Arch Gen Psychiatry 63, 1026–1032.
Mieusset, Roger, Bourras Bengoudifa, and Louis Bujan. "Effect of posture and clothing on scrotal temperature in fertile men." Journal of Andrology 28.1 (2007): 170-175.
Haj, M., et al. "Effect of external scrotal cooling on the viability of the testis with torsion in rats." European Surgical Research 39.3 (2007): 160-169.
Jung, A., and H‐C. Schuppe. "Influence of genital heat stress on semen quality in humans." Andrologia 39.6 (2007): 203-215.
Ivell, Richard. "Lifestyle impact and the biology of the human scrotum." Reprod Biol Endocrinol 5.15 (2007): 1477-1482.
Hammoud et al, 2008. Impact of male obesity on infertility: a critical review of the current literature. Journal of Fertility and Sterility, 2008 Oct;90(4):897-904.
Hansen, Peter J. "Effects of heat stress on mammalian reproduction." Philosophical Transactions of the Royal Society B: Biological Sciences 364.1534 (2009): 3341-3350.
Owen et al, 2010. Sedentary Behavior: Emerging Evidence for a New Health Risk. Mayo Clinic Proceedings, 2010 Dec; 85(12): 1138–1141.
Phillips and Tanphaichitr, 2010. Mechanisms of obesity-induced male infertility. Expert review Endocrinology & Metabolism. March 2010. 5(2):229-251.
Ferlin, Alberto, et al. "Heat shock protein and heat shock factor expression in sperm: relation to oligozoospermia and varicocele." The Journal of Urology 183.3 (2010): 1248-1252
Parviz Gharagozloo, R. John Aitken, The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy, Human Reproduction, Volume 26, Issue 7, 1 July 2011, Pages 1628–1640
Sheynkin, Yefim, et al. "Protection from scrotal hyperthermia in laptop computer users." Fertility and Sterility 95.2 (2011): 647-651.
Menkveld et al, 2011. Measurement and significance of sperm morphology. Asian Journal of Andrology, 2011 Jan; 13(1): 59–68.
Vaziri MH, Sadighi Gilani MA, Kavousi A, Firoozeh M, Khani Jazani R, Vosough Taqi Dizaj A, Mohseni H, Bagery Lankarani N, Azizi M, Salman Yazdi R. The Relationship between Occupation and Semen Quality. Int J Fertil Steril. 2011 Jul;5(2):66-71. Epub 2011 Sep 23. PMID: 24963361; PMCID: PMC4059951.
Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, Kirkman-Brown J, Coomarasamy A. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012 Oct;27(10):2908-17. doi: 10.1093/humrep/des261. Epub 2012 Jul 12. PMID: 22791753.
Sharpe, 2012. Sperm counts and fertility in men: a rocky road ahead. EMBO Reports, 2012 May; 13(5): 398–403.
Jenkins and Carrell, 2012. The sperm epigenome and potential implications for the developing embryo. Journal of Reproduction, Jun 2012.
Gannon et al, 2014 The Sperm Epigenome: Implications for the Embryo. Advances in Experimental Medicine and Biology, January 2014.
Garolla et al, 2015. Twenty-four-hour monitoring of scrotal temperature in obese men and men with a varicocele as a mirror of spermatogenic function. Journal of Human Reproduction, Volume 30, Issue 5, 1 May 2015, Pages 1006–1013.
Coughlan C, Clarke H, Cutting R, Saxton J, Waite S, Ledger W, Li T, Pacey AA. Sperm DNA fragmentation, recurrent implantation failure and recurrent miscarriage. Asian J Androl. 2015 Jul-Aug;17(4):681-5. doi: 10.4103/1008-682X.144946. PMID: 25814156; PMCID: PMC4492063.
Katib, 2015. Mechanisms linking obesity to male infertility. Central European Journal of Urology, 2015; 68(1): 79–85.
Rex AS, Aagaard J, Fedder J. DNA fragmentation in spermatozoa: a historical review. Andrology. 2017 Jul;5(4):622-630. doi: 10.1111/andr.12381. PMID: 28718529; PMCID: PMC5601286.
Anaissie J, DeLay KJ, Wang W, Hatzichristodoulou G, Hellstrom WJ. Testosterone deficiency in adults and corresponding treatment patterns across the globe. Transl Androl Urol. 2017 Apr;6(2):183-191. doi: 10.21037/tau.2016.11.16. PMID: 28540225; PMCID: PMC5422691.
Levine et al, 2017. Temporal trends in sperm count: a systematic review and meta-regression analysis. Human Reproduction Update, Volume 23, Issue 6, 1 November 2017, Pages 646–659.
Liu & Ding, 2017. Obesity, a serious etiologic factor for male subfertility in modern society. Journal of Reproduction, 2017 Oct;154(4):R123-R131.
Kahn & Brannigan, 2017. Obesity and male infertility. Current Opinion in Urology, 2017 Sep;27(5):441-445.
Braun et al, 2017. Fathers Matter: Why It's Time to Consider the Impact of Paternal Environmental Exposures on Children's Health. Current Epidemiological Reports. March 2017.
Rim Frikha, Taoufik Frikha, Nouha Bouayed, Tarek Rebai, Assessment of male factor involved in recurrent pregnancy loss: A preliminary study, Middle East Fertility Society Journal, Volume 23, Issue 3, 2018, Pages 238-240, ISSN 1110-5690
Mínguez-Alarcón L, Gaskins AJ, Chiu YH, Messerlian C, Williams PL, Ford JB, Souter I, Hauser R, Chavarro JE. Type of underwear worn and markers of testicular function among men attending a fertility center. Hum Reprod. 2018 Sep 1;33(9):1749-1756. doi: 10.1093/humrep/dey259. PMID: 30102388; PMCID: PMC6530653.
Rahman et al, 2018 Heat stress responses in spermatozoa: Mechanisms and consequences for cattle fertility. Theriogenology, June 2018.
Turner KA, Rambhatla A, Schon S, Agarwal A, Krawetz SA, Dupree JM, Avidor-Reiss T. Male Infertility is a Women's Health Issue-Research and Clinical Evaluation of Male Infertility Is Needed. Cells. 2020 Apr 16;9(4):990. doi: 10.3390/cells9040990. PMID: 32316195; PMCID: PMC7226946.
Christopher L R Barratt, Christopher J De Jonge, Richard A Anderson, Michael L Eisenberg, Nicolás Garrido, Satu Rautakallio Hokkanen, Csilla Krausz, Sarah Kimmins, Moira K O’Bryan, Allan A Pacey, Frank Tüttelmann, Joris A Veltman, A global approach to addressing the policy, research and social challenges of male reproductive health, Human Reproduction Open, Volume 2021, Issue 1, 2021, hoab009
Benidir T, Remondini T, Lau S, Jarvi KA. Evaluation of patient compliance with the use of scrotal cooling devices. F S Rep. 2021 Jun 30;2(3):289-295. doi: 10.1016/j.xfre.2021.06.007. PMID: 34553153; PMCID: PMC8441567.
Ferlin, A., Garolla, A., Ghezzi, M., Selice, R., Palego, P., Caretta, N., Di Mambro, A., Valente, U., De Rocco Ponce, M., Dipresa, S., Sartori, L., Plebani, M., & Foresta, C. (2021). Sperm Count and Hypogonadism as Markers of General Male Health. European Urology Focus, 7(1), 205-213. https://doi.org/10.1016/j.euf.2019.08.001
Malathi A, Iyer RP, Mohan R, Balakrishnan S. Impact of Seasonal Variations on Semen Parameters: A Retrospective Analysis of Data from Subjects Attending a Tertiary Care Fertility Centre. J Hum Reprod Sci. 2023 Apr-Jun;16(2):114-120. doi: 10.4103/jhrs.jhrs_20_23. Epub 2023 Jun 30. PMID: 37547085; PMCID: PMC10404019.
Zhao C, Sun L, Zhao P. Effects of sperm processing techniques on IVF pregnancy rates: a mini-review. Ther Adv Reprod Health. 2023 Jul 23;17:26334941231188656. doi: 10.1177/26334941231188656. PMID: 37497119; PMCID: PMC10366343.
Mínguez-Alarcón L, Williams PL, Souter I, Ford JB, Ghayda RA, Hauser R, Chavarro JE; Earth Study Team. Occupational factors and markers of testicular function among men attending a fertility center. Hum Reprod. 2023 Apr 3;38(4):529-536. doi: 10.1093/humrep/dead027. PMID: 36772979; PMCID: PMC10068265.
Global, regional and national burden of male infertility in 204 countries and territories between 1990 and 2019: an analysis of global burden of disease study. BMC Public Health. Available at: BMC Public Health (BioMed Central).
Aetiological and epidemiological features of male infertility. Human Reproduction Update. Available at: Oxford Academic (Oxford Academic).
“We considered ourselves healthy but our sperm results were low and slow.”
Global fertility rate (livebirths per woman)
Global Trends: The Male Fertility Crisis
Male infertility is a growing global issue, affecting 1 in 6 adults worldwide (PAHO/WHO, 2023). Since 1990, male infertility has surged by 76.9%, outpacing population growth, with the highest rates in Africa and Eastern Europe.
At the same time, global birth rates have plummeted, with more than half of all countries now below population replacement levels. By 2050, over 75% of nations won’t have enough births to sustain their populations, and by 2100, this will rise to 97% (The Lancet, 2020).
Beyond personal struggles, declining fertility has massive economic and societal consequences, from shrinking workforces to unsustainable social security systems. Some experts even warn that low birth rates could impact national security and geopolitical power.